Abstract
Introduction: In August 2019, fedratinib (FEDR) became the second treatment (in addition to ruxolitinib [RUX]) to be approved for intermediate- or high-risk primary or secondary myelofibrosis (MF). No real-world data (RWD) have been presented on demographics, clinical characteristics, and treatment patterns of FEDR for patients receiving FEDR per US label post RUX failure.
Methods: Adults with intermediate- or high-risk MF who initiated FEDR on or after August 16, 2019 (FEDR approval date) after discontinuing RUX, with ≤ 90 days of follow-up post FEDR initiation (and having completed ≥1 cycle of FEDR) and who had a spleen size assessment at initiation of FEDR, were identified from community oncology practices in the USA. The patients' treating physicians selected eligible patients and completed electronic case report forms. Providers abstracted patient clinical characteristics at diagnosis and during RUX and FEDR therapy, including bone marrow fibrosis grade, biomarkers, risk score, performance status, spleen size (centimeters above the costal margin), symptoms (listing per the Modified Myelofibrosis Symptom Assessment Form version 4.0), and complete blood count data. Dose, dose modifications (reductions, interruptions, or increases), and dates/rationale for RUX treatment failure per provider report were collected. Primary reasons for RUX failure were designated a priori and included refractory or suboptimal response, disease progression, or intolerance to RUX therapy.
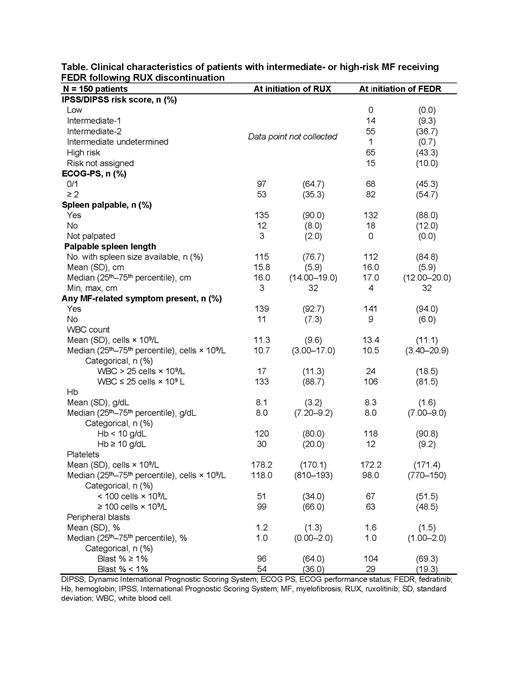
Results: For 150 eligible patients, median age at diagnosis was 68 years, 55% of patients were male, 68% were non-Hispanic White, 85% had primary MF, 55% were JAK2 V617F positive, and 9% were JAK2/MPL/CALR negative. Dose at RUX initiation was ≥ 20 mg twice a day (bid) for 50% of patients. At RUX initiation, 65% of patients had ECOG 0/1 scores, and 90% had palpable spleen. Median platelet count was 118 ×10 9/L.
Dose reductions during RUX were reported in 13% (n = 19), treatment interruptions in 3%, and dose increases in 12% of patients. Rationale for reductions were neutropenia (7/19), thrombocytopenia (6/19), other (3/19), patient request (2/19), and anemia (1/19). Dose increases were due to titration to therapeutic dose (13/18) and persistent MF symptoms (5/18). Interruptions were due to thrombocytopenia (2/4), neutropenia (1/4), and patient request (1/4). Overall 37% of patients experienced an adverse event while on RUX including thrombocytopenia (25 total; 4 ≥ grade 3), anemia (24 total, 3 ≥ grade 3), fatigue (24 total, 1 ≥ grade 3) and nausea (10 total, 1 ≥ grade 3). Primary rationale for discontinuation of RUX was disease progression (70%), refractory or suboptimal response (25%), and intolerance (5%). Among those with refractory response, providers most commonly reported < 25% reduction of spleen size as the indicator of refractory disease (46%), no improvement in MF symptoms or quality of life (32%), and other reasons (22%). Among patients deemed to have progression as the rationale for RUX discontinuation, the most common reason cited was return of MF symptoms (65%), increase of spleen size to > 50% versus best achieved response (23%), and other reasons (12%). Median duration of RUX was 4.4 (range 1-21) months among refractory or intolerant patients, and 14 (range 2-65) months among those with disease progression.
FEDR was initiated at 400 mg bid for 74% of patients. At FEDR initiation, 43.3% of patients had International Prognostic Scoring System (IPSS)/ Dynamic IPSS (DIPSS) High-risk, 36.7% Intermediate (Int)-2 risk, 9.3% Int-1, and 10.0% unknown risk (Table). At initiation of FEDR, 88% of patients had palpable spleen, mean spleen size was 16 cm, and median platelet count was 98 × 10 9/L (Table 1). Symptoms reported at FEDR initiation included fatigue (72%), abdominal discomfort (51%), night sweats (44%), early satiety (25%), bone pain (18%), itching (13%), and pain under the left rib (13%). Dose modifications were observed for 6% of patients while receiving FEDR during follow-up.
Conclusions: This study represents the first RWD description of patient characteristics and treatment journey from MF diagnosis through RUX therapy and FEDR treatment. Patients had shorter duration of RUX therapy and had poorer spleen and blood counts at the time of FEDR initiation compared with at initiation of RUX. Patients at risk of early failure of RUX should be considered for earlier intervention with FEDR.
Harrison: CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sierra Oncology: Honoraria; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Corporation: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Constellation Pharmaceuticals: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mascarenhas: Galecto: Consultancy; Prelude: Consultancy; Geron: Consultancy; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Geron: Consultancy, Research Funding; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forbius: Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Promedior: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merus: Research Funding. Abraham: Bristol Myers Squibb: Current Employment. Kee: Bristol Myers Squibb: Current Employment. Nadal: Bristol Myers Squibb: Current Employment. Balanean: Cardinal Health: Current Employment; Georgia State University: Other: former student and employee. McBride: BMS: Current Employment. Kish: Cardinal Health: Current Employment, Current equity holder in publicly-traded company, Research Funding. Liassou: Cardinal Health: Current Employment. Feinberg: Cardinal Health: Current Employment. Gerds: PharmaEssentia Corporation: Consultancy; Novartis: Consultancy; Sierra Oncology: Consultancy; AbbVie: Consultancy; Celgene/Bristol Myers Squibb: Consultancy; Constellation: Consultancy; CTI BioPharma: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal